ACTRIS Marks a New Chapter in CGT with the Launch of Its National Facility
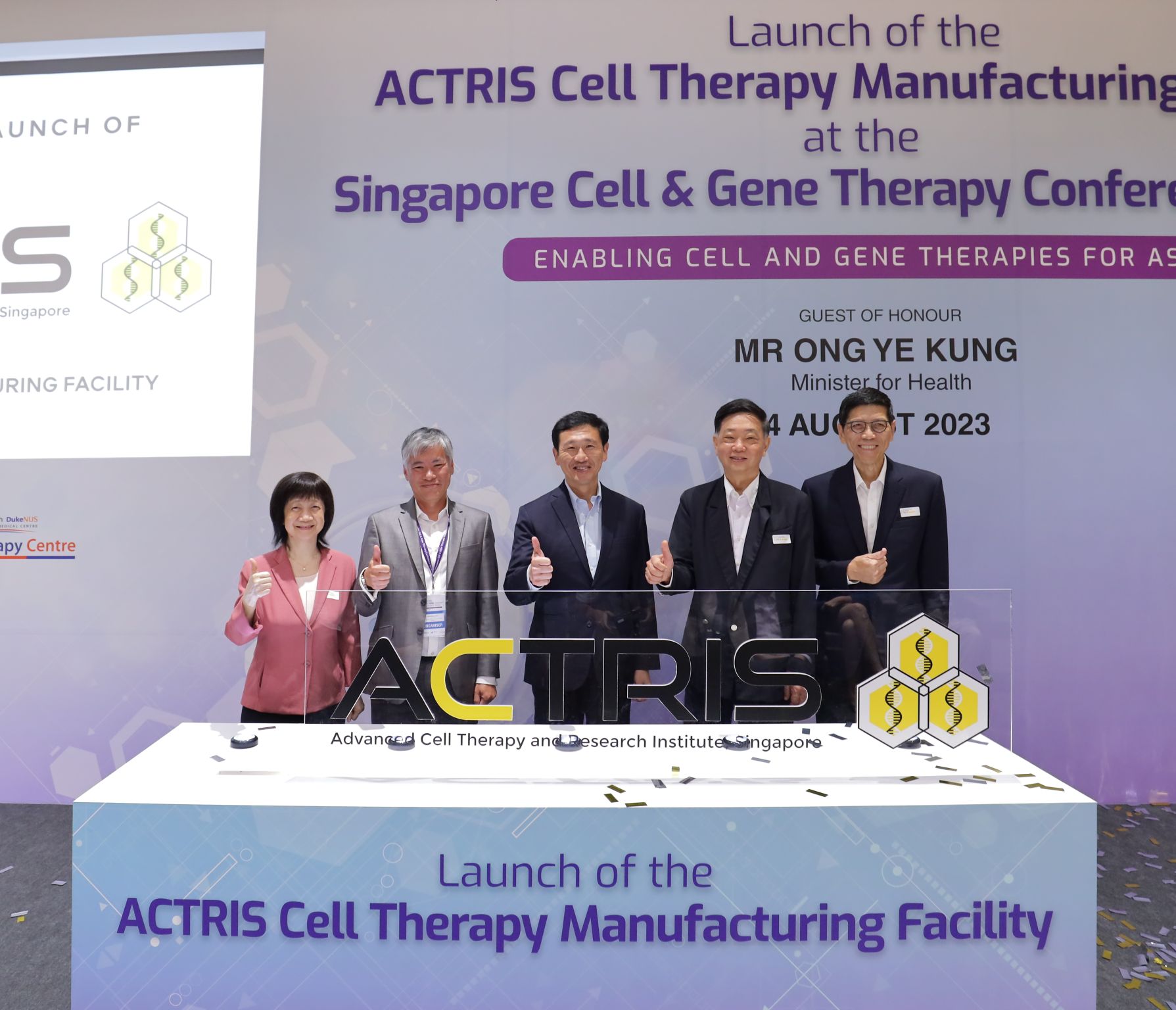
We launched our new cell therapy facility this morning! 🎉
With 14 GMP-compatible suites, four translational labs and one quality
control lab, it is the largest national facility of its kind which will
support hospital services, academic institutions for research, and biotech
start-ups, to meet the clinical demand for cell and gene therapy treatments
in Singapore.
The facility was launched by Minister for Health Mr Ong Ye Kung this morning,
in conjunction with the inaugural Singapore Cell and Gene Therapy Conference.
“We look forward to contributing ground-breaking work that will advance
cancer treatment and regenerative medicine. In addition, ACTRIS will facilitate
more public-private partnerships in the future and help companies looking
to develop their CGT products to obtain the required regulatory approvals
to bring their cell therapy products into Singapore,” said A/Prof Danny Soon,
Interim Executive Director of ACTRIS and CEO of Consortium for Clinical Research and Innovation, Singapore (CRIS).
He added: “It takes a village to raise Singapore’s capacity for CGT and
we are excited to collaborate with our academic institutes, hospital clusters,
local biotechnology and pharmaceutical industry partners. This facility
will be a welcome boost to the CGT ecosystem in Singapore and the region.”
Our full press release is available at here.
Check out highlights of the launch event below!


