Milestones & Achievements
🏆 2025: Certification & First Delivery
From Vision to Patient Impact
Following its first Health Sciences Authority (HSA) inspection, ACTRIS achieved Good Manufacturing Practice (GMP) Certification and received a Manufacturer’s Licence for investigational and specially authorised cell therapy products for use in local hospitals.
In July 2025, ACTRIS successfully delivered its first product under the innovative salvage therapy framework that brought renewed hope and extended care to a patient in need.
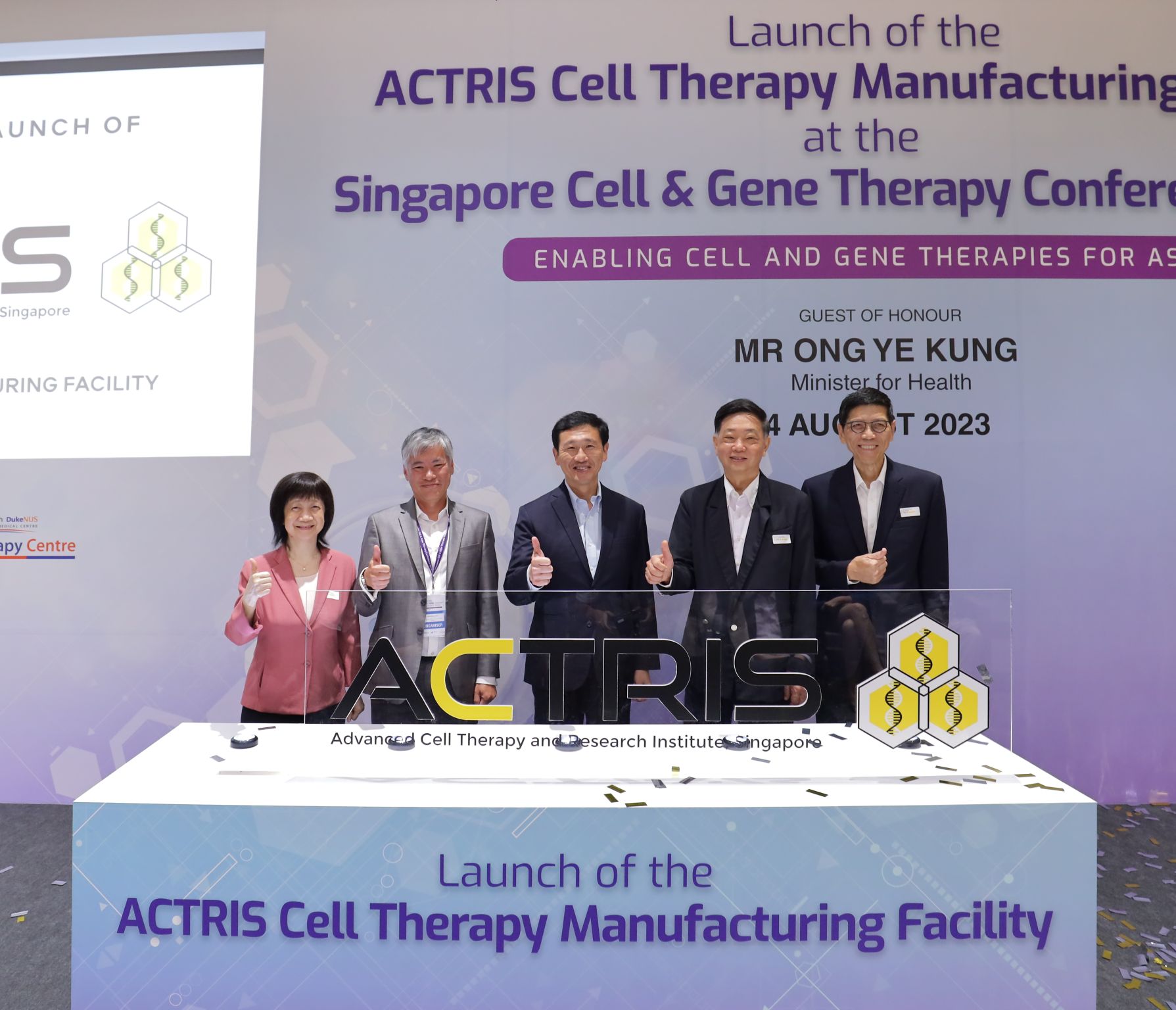
🚀 2023 - 2024: National Launch & Expansion
Official Launch & Early Impact
On 23 August 2023, the Minister for Health officially launched the ACTRIS facility — Singapore’s largest national manufacturing facility for CGT — affirming its role as a cornerstone of the nation’s growing CGT ecosystem.
ACTRIS began onboarding early collaborative projects to support the manufacture of investigational CGT products.
ACTRIS secured RIE2025 funding to advance research translation, enhance process development capabilities with A*STAR Bioprocessing Technology Institute (BTI), and expand manufacturing of investigational CGT products.
🛠️ 2021 - 2022: Resilence & Readiness - Facility Comes to Life
Constructing Capabilities: ACTRIS Advances amidst Global Challenges
Despite the global disruption of COVID-19, ACTRIS pressed on — designing and constructing a state-of-the-art facility, installing specialised equipment, and strengthening awareness of its role within Singapore’s CGT ecosystem.
ACTRIS secured baseline operational funding to recruit and train critical expertise and commission the facility essential for national readiness.
🔬 2019 - 2020: Infrastructure & Identity - ACTRIS Emerges
Turning Plans into National Capabilities
As plans matured, the focus turned to capability building. NCTC IO secured government funding for infrastructure — paving the way for a national facility to accelerate CGT development and access.
Building on this foundation, NCTC evolved into ACTRIS — the Advanced Cell Therapy and Research Institute, Singapore — and on 20 April 2020, became part of the Consortium for Clinical Research and Innovation, Singapore (CRIS).
🏗️ 2017 - 2018: Strategic Foundations - Building the Framework
Establishing the NCTC Implementation Office: Strategy Meets Structure
To translate this vision into action, the NCTC Implementation Office (IO) was established to define strategy, governance, and resource requirements. The IO laid a strong foundation to enable research translation and enhance clinical access to CGTs.
🌱 2015 - 2016: Vision Ignited - Laying the Groundword for CGT in Singapore
From Vision to Blueprint: The Birth of National Cell Therapy Centre
It began with a vision — to make cell and gene therapy (CGT) a reality for patients in Singapore and beyond. Guided by the Ministry of Health’s (MOH) National Cell Therapy Services Advisory Committee, the concept of a National Cell Therapy Centre (NCTC) took shape through extensive horizon scanning, global benchmarking, and strategic engagement with hospitals, research institutes, and industry partners.

